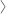Description
Now you can standardise your molecular assays for parasitic diseases such as malaria, with a new range of synthetic controls that provide stable, consistent, safe and abundant material.The new Simply Molecular® G-Sphere® range of standards from Phthisis Diagnostics, exclusively available in the UK from Alpha Laboratories, contain double stranded DNA gene(s). These have been designed and synthetically created for use as genetic surrogate control material in molecular applications such as PCR.
Ready to use, G-Sphere Molecular Standards are non-hazardous and eliminate the need to extract DNA from potentially infectious samples.
Unlike home-made controls, the G-Sphere Molecular Standards are produced in accordance with FDA cGMP regulations to ensure consistent quality and reproducibility. These genetic surrogates are also stable for up to one year when stored at -20C.
The G-Sphere range includes Molecular Standards targeted against the malaria causing Plasmodium parasites; P falciparum, P.ovale, P. malariae and P. vivax. There are also G-Sphere standards for 12 species of Cryptosporidium, from C. baileyi to C. wrairi and other intestinal parasites such as Giardia, Entamoeba histolytica, E. dispar and microsporidia.
These molecular standards are a perfect complement to the E-Sphere® Simple NA kit. This easy to use extraction method for total nucleic acids is suitable for use with a variety of sample material, liquid or semi-solid, including body fluids, culture media, environmental samples, swabs and tissues (fresh/frozen/formalin fixed paraffin embedded).